The activation energy equation using the arrhenius formula is: Substracting equation (4) from equation (3) results in rerrangement of equation (5) and solving for e a yields If we look at the equation that this arrhenius equation calculator uses, we can try to understand how it works: It provides insight into the dependence of. Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant.
Web find the activation energy. If we look at the equation that this arrhenius equation calculator uses, we can try to understand how it works: Web for this purpose, the rate constant is measured for two different temperatures t 1 and t 2. Web the arrhenius equation relates the activation energy and the rate constant, k, for many chemical reactions:
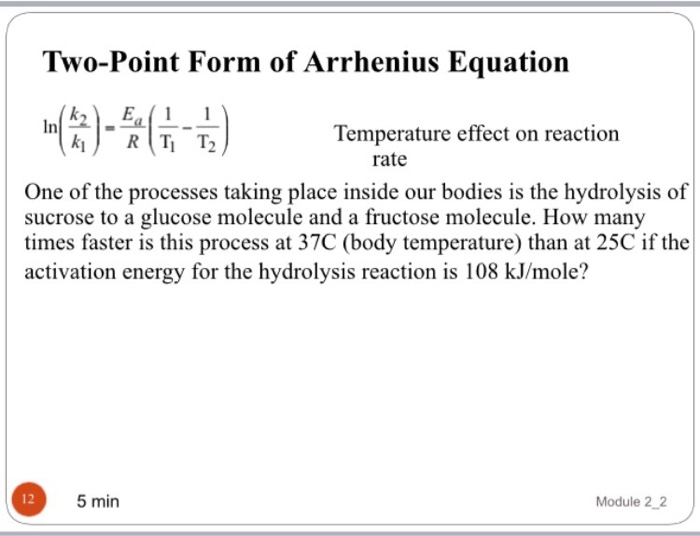
On rearranging the equation, an expression for the activation energy is generated. 126 views 5 years ago general chemistry. Web the arrhenius equation relates the activation energy and the rate constant, k, for many chemical reactions:
The activation energy, e a, is the minimum energy molecules must possess in order to react to form a product. Web the arrhenius equation as it's used in chemistry is often stated according to the formula: Web the arrhenius equation, k = ae − ea / rt. The slope of the arrhenius plot can be used to find the activation energy. 1.1k views 3 years ago general.
Web two point form of the arrhenius equation? Want to join the conversation? How to write different forms of the arrhenius equation.
Want To Join The Conversation?
On rearranging the equation, an expression for the activation energy is generated. Substracting equation (4) from equation (3) results in rerrangement of equation (5) and solving for e a yields If we look at the equation that this arrhenius equation calculator uses, we can try to understand how it works: A is an exponential factor that is a constant for a given chemical reaction, relating the frequency of collisions of particles.
Using The Arrhenius Equation To Look At How Changing Temperature And Activation Energy Affects Collisions.
K = ae − ea / rt. Web forms of the arrhenius equation (video) | khan academy. Web the arrhenius equation gives the dependence of the rate constant of a chemical reaction on the absolute temperature as. Can be visualized as the frequency of correctly oriented collisions between reactant particles).
The Activation Energy, E A, Is The Minimum Energy Molecules Must Possess In Order To React To Form A Product.
Web the arrhenius equation as it's used in chemistry is often stated according to the formula: Web two point form of the arrhenius equation? Can be visualised as the frequency of correctly oriented collisions between reactant particles). If the rate constant doubles, for example, so does the rate of the reaction.
The Slope Of The Arrhenius Plot Can Be Used To Find The Activation Energy.
Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. By substituting any two data pairs and further calculation yields the value for the activation energy in joules per mole or kilojoules per mole. E a is the activation energy of the reaction (usually given in joules per mole or j/mol) Web the arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction.
Web forms of the arrhenius equation (video) | khan academy. Can be visualised as the frequency of correctly oriented collisions between reactant particles). How to write different forms of the arrhenius equation. If we look at the equation that this arrhenius equation calculator uses, we can try to understand how it works: This gives less accurate values for e a, but is computationally quicker.