Knowing this lets us use the periodic table to identify the element as al (aluminum). Web the energy released when an electron is added to the neutral atom and a negative ion is formed. Compounds formed from positive and negative ions are ionic compounds. Therefore, the valency and valence electrons of aluminum are 3. Define ion, cation, and anion.
Electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative scale. The following table shows how we name the ions for the first four elements in this group: Recognize characteristics of monatomic and polyatomic ions. Al 3 +, an aluminum ion.
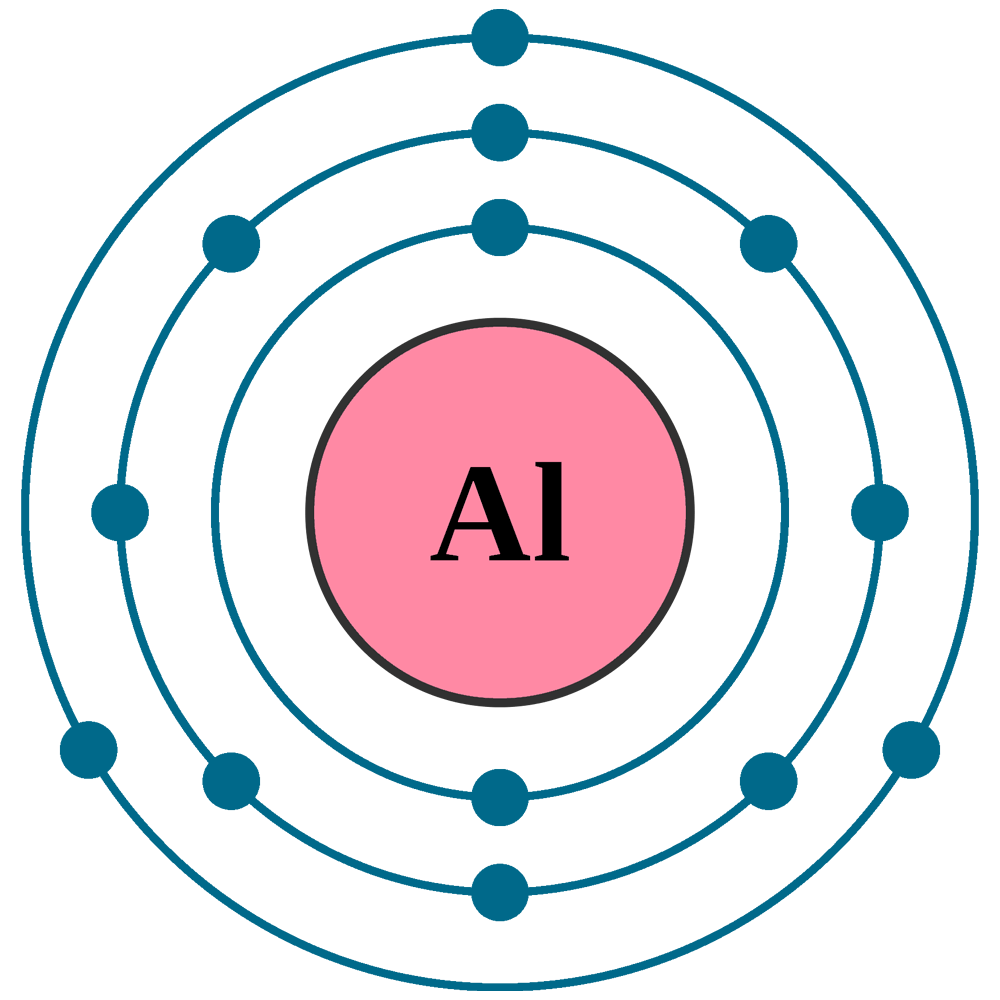
The difference between the mass number of the aluminum atom and the number of protons is fourteen. For main group elements, the electrons that were added last are the first electrons removed. Al 3 +, an aluminum ion.
Since aluminum's atomic number is thirteen, it has thirteen electrons. Predict which forms an anion, which forms a cation, and the charges of each ion. Electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative scale. How do we name main group cations? The ion that is formed is_al³⁺_____.
Define ion, cation, and anion. Determine the number of subatomic particles in an ion. Here is my way of remembering which charge is with anion and cation:
Web Chemistry For Allied Health (Soult) 2:
Most atoms do not have eight electrons in their valence electron shell. A proton never moves from one atom to another. You then split the electrons between the different orbitals. Web the energy released when an electron is added to the neutral atom and a negative ion is formed.
When They Do, They Become Monatomic Ions.
Transition and inner transition metal elements behave differently than main group elements. Web because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Web remember that ions are formed only when electrons move from one atom to another; Electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative scale.
The Al Atom Has Lost Three Electrons And Thus Has Three More Positive Charges (13) Than It Has Electrons (10).
C 4−, a carbide ion. The ion that is formed is al³⁺.explanation:an atom of aluminum has 13 electrons in its neutral state. Knowing this lets us use the periodic table to identify the element as al (aluminum). Determine the number of subatomic particles in an ion.
Define Ion, Cation, And Anion.
38 people found it helpful. In this video we’ll use the periodic table and a few simple rules to find the number of protons and electrons for the. Ions form when atoms lose or gain. Here is my way of remembering which charge is with anion and cation:
Define ion, cation, and anion. Therefore, an aluminum atom has fourteen neutrons. After the electron configuration, the last shell of the aluminum atom has three electrons. The difference between the mass number of the aluminum atom and the number of protons is fourteen. ( 20 +) + ( 18 −) = 2 +.