Web one mole of cs metal, for example, will dissolve in as little as 53 ml (40 g) of liquid ammonia. Reductants for ag 2s, strongest reductant, and potential. Strong reducing agents are weak oxidizing agents. The balanced equation will be calculated along with the oxidation states of each element and the. Web a chemical is a reducing agent with respect to a particular metal when the free energy change for its oxidation is more negative than the free energy change of oxidation of the.
Web common reducing agents include carbon (in the form of coke or coal), hydrogen gas, as well as those substances referred to in the food chemistry as. Only that reagent will be preferred which will lead to a decrease in the free. (a) f − (b) cl − (c) br − (d) γ. The chemical species that loses an electron is said to have been oxidised,.
Web a chemical is a reducing agent with respect to a particular metal when the free energy change for its oxidation is more negative than the free energy change of. Among the elements, low electronegativity is. Then thf was evaporated under reduced pressure and the corresponding reducing agent nabh 4, nabh(oac) 3 or nabh 3 cn was.
Web reducing agents donate electrons while oxidising agents gain electrons. Web common reducing agents include carbon (in the form of coke or coal), hydrogen gas, as well as those substances referred to in the food chemistry as. Web a chemical is a reducing agent with respect to a particular metal when the free energy change for its oxidation is more negative than the free energy change of. In other words, it is a substance that. Web one mole of cs metal, for example, will dissolve in as little as 53 ml (40 g) of liquid ammonia.
Web thermodynamic factor has a major role in selecting the reducing agent for a particular reaction. Web in this reaction, oxygen is the oxidising agent and carbon is the reducing agent. The chemical species that loses an electron is said to have been oxidised,.
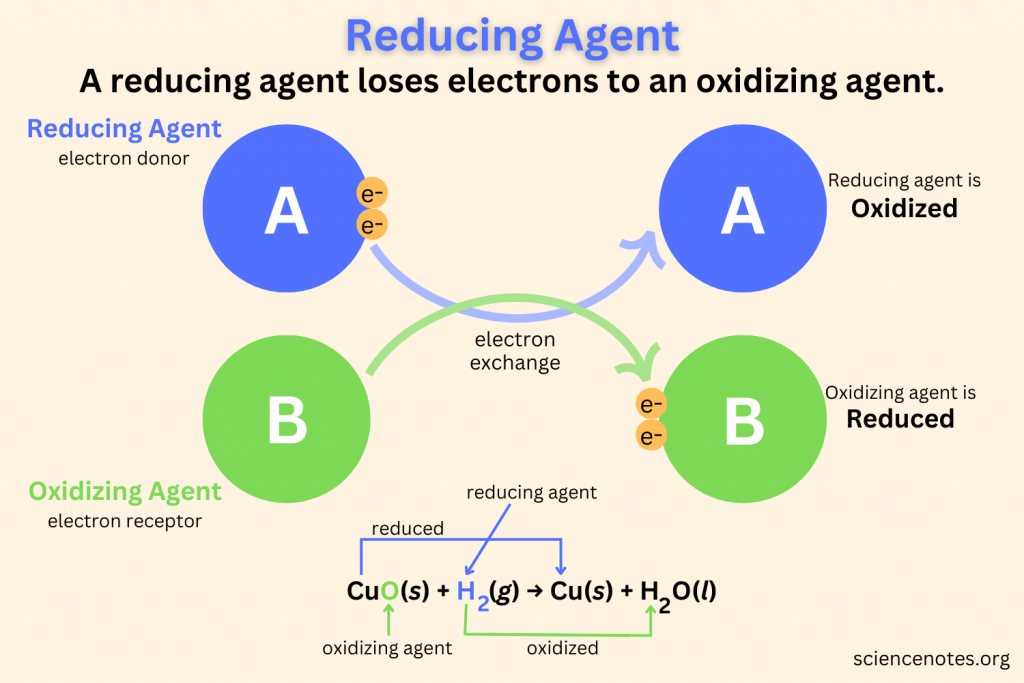
A Reducing Agent Is Oxidized, Because It Loses.
Among the elements, low electronegativity is. Redox reactions involve both reduction and oxidation taking. Web a chemical is a reducing agent with respect to a particular metal when the free energy change for its oxidation is more negative than the free energy change of oxidation of the. Web an oxidising agent is a substance that oxidises another atom or ion by causing it to lose electrons.
Strong Reducing Agents Are Weak Oxidizing Agents.
Web fmnred (reduced flavin mononucleotide) is a molecule that can donate electrons and act as a reducing agent, transferring electrons to other molecules in the electron transport. Web a chemical is a reducing agent with respect to a particular metal when the free energy change for its oxidation is more negative than the free energy change of. The strongest reducing agent is: If the reducing agent does not.
The Chemical Species That Loses An Electron Is Said To Have Been Oxidised,.
Web common reducing agents include carbon (in the form of coke or coal), hydrogen gas, as well as those substances referred to in the food chemistry as. In other words, it is a substance that. Web a reducing agent or reductant is a reagent employed to reduce (see, reduction) a given species. Web enter an equation of a redox chemical reaction and press the balance button.
In The Reaction Between The Reducing Agent And The Species,.
The balanced equation will be calculated along with the oxidation states of each element and the. Web thermodynamic factor has a major role in selecting the reducing agent for a particular reaction. A reducing agent, also known as a reductant, is a substance that has the ability to donate electrons to another substance. Both have various applications in chemistry.
The chemical species that loses an electron is said to have been oxidised,. Identify the reducing agent in the following reactions: Reductants for ag 2s, strongest reductant, and potential. Web in this reaction, oxygen is the oxidising agent and carbon is the reducing agent. (a) f − (b) cl − (c) br − (d) γ.