8 electrons in the second shell. Try this interactive simulation and explore the structure and symbols of atoms, isotopes, and ions. Sharp (s), principal (p), diffuse (d), and fundamental (f). Sodium (na) has atomic number 11, hence, 11 electrons. If you’ve already watched the video, click here, or scroll down below the video to start interacting.
8 electrons in the second shell. The electronic structure of an atom can be predicted from its atomic number. Try this interactive simulation and explore the structure and symbols of atoms, isotopes, and ions. Then play a game to test your ideas!
An ionic compound is made up of charged particles, called ions. The number of protons is always equal to. The diagrams show two ways of representing this electron transfer.
An ionic compound is made up of charged particles, called ions. 8 electrons in the second shell. Then play a game to test your ideas! I’ve created an interactive app that will draw atoms (of the first 20 elements), to go with a worksheet for student practice. We draw atomic structures for any element with the help of atomic number they have.
If you’ve already watched the video, click here, or scroll down below the video to start interacting. How many core electrons are there? 2 electrons in the first shell.
I’ve Created An Interactive App That Will Draw Atoms (Of The First 20 Elements), To Go With A Worksheet For Student Practice.
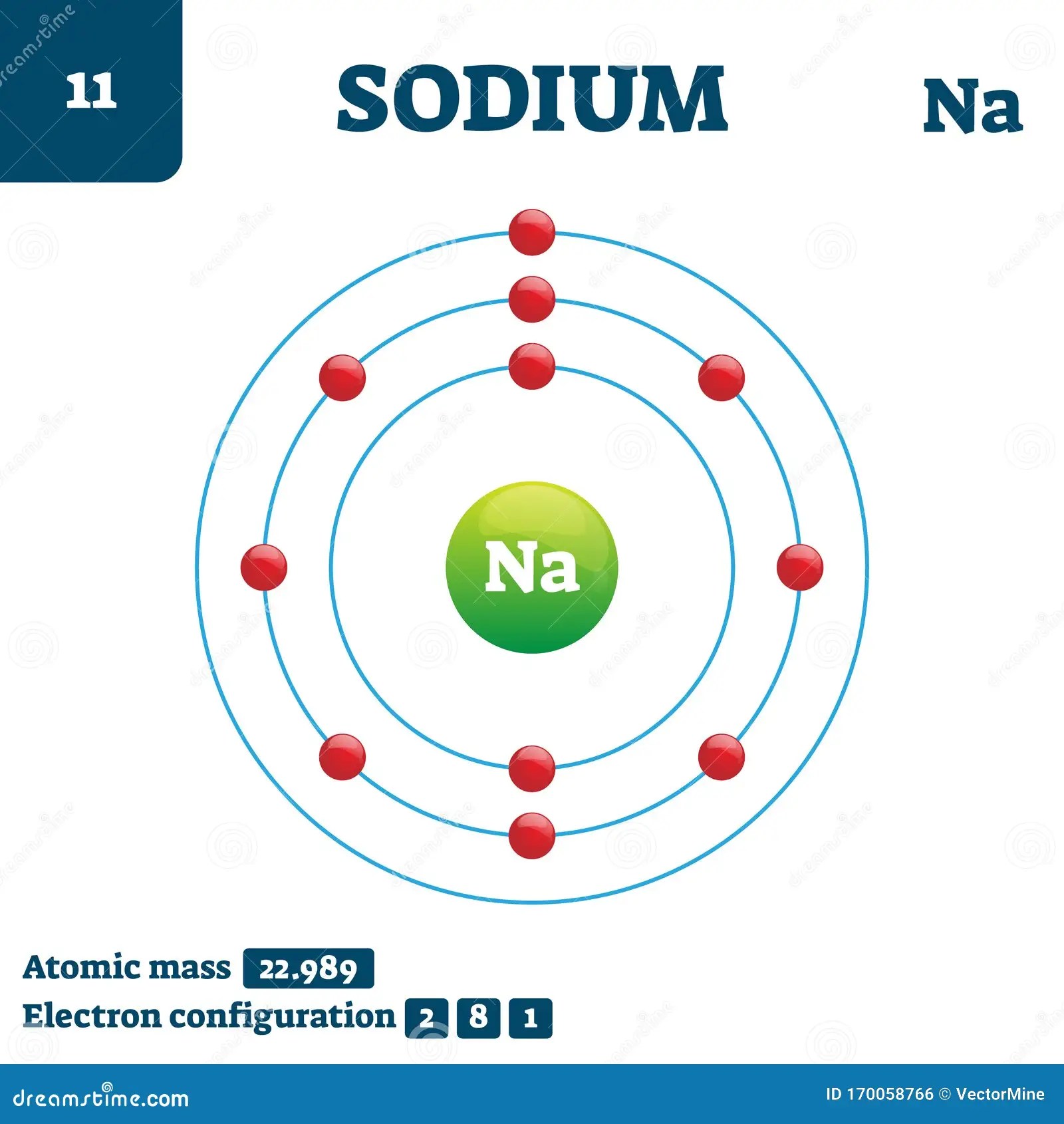
Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then. 2 electrons in the first shell. Web to draw the sodium bohr model, outline the 11 protons, 12 neutrons, and 11 electrons. You can also play a fun game to check your understanding of atomic concepts.
Here, You’ll Learn How To Determine An Atom’s Specific Structure, Which Will Set Us Up For Learning About Chemical Bonds In The Next Tutorials.
Web exercise \(\pageindex{2}\) counting valence electrons in sodium atoms. In this video we'll look at the atomic. An ionic compound is made up of charged particles, called ions. The sodium atom (na) is commonly used for examples and practice problems in chemistry.
Sodium (Na) Has Atomic Number 11, Hence, 11 Electrons.
Try this interactive simulation and explore the structure and symbols of atoms, isotopes, and ions. I show you where sodium is on the periodic table and how to determine how many valence electrons it has. A picture of a hydrogen atom can be found here. It is in group 1 of the periodic table.
Web This Video Shows How To Draw The Orbital Diagram Of Sodium (Na).
The electronic structure of an atom can be predicted from its atomic number. Web for example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. Blockelements are organised into blocks by the orbital type in which the outer electrons are found. It has a giant lattice structure with strong electrostatic forces of attraction.
How many core electrons are there? The diagrams show two ways of representing this electron transfer. These blocks are named for the characteristic spectra they produce: Sodium (na) has atomic number 11, hence, 11 electrons. How do you draw the shell diagram of sodium atom?




:max_bytes(150000):strip_icc()/sodiumatom-58b602715f9b5860464c7a22.jpg)
