Magnesium + hydrogen sulfate æ 3. In this general reaction, element a a is a metal and replaces element b b (also a metal) in the compound. Carry out single replacement reactions. Cl 2 + ki → 4. Web worksheet on single & double replacement reactions predict the products.
Web single replacement reaction worksheet for each reaction use the activity series to complete the reaction. The starting materials are always pure elements like hydrogen gas or a pure zinc metal, plus an aqueous compound. Predicting single replacement reactions for each of the following reactions, • predict whether or not the reaction will occur. A single replacement reaction or a single displacement reaction is defined as a reaction in which one element is superseded by another element in a compound.
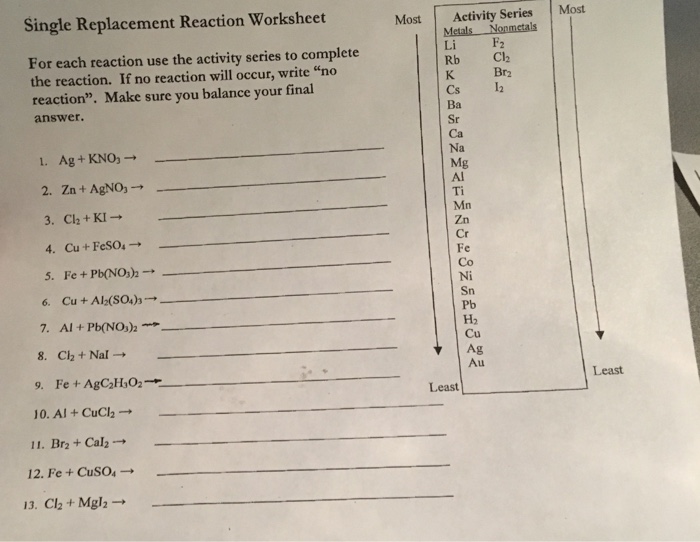
If no single replacement reaction occurs, write nr to the right of the arrow. Be sure to balance each equation. Ag + kno 3 → 2.
Web the equation for the reaction is: Cl 2 + ki → 4. Use the activity series to determine this. If there is no reaction, then just put no rxn. The general equation is a + bc → ac + b examples are zn + 2hcl → zncl₂ + h₂, where zn replaces h in hcl, and f₂.
Cu + feso 4 → 5. Web chemistry single replacement reaction worksheet. Cu + al 2(so 4)
If No Reaction Will Occur, Write “No Reaction”.
Students will learn to use the activity series for the first time as part of this worksheet, as. Web chemistry single replacement reaction worksheet. Li is above mg on the chart 2. In this general reaction, element a a is a metal and replaces element b b (also a metal) in the compound.
List Metals In Order Of Activity Based On Your Observation.
Web chemistry single replacement reaction worksheet 1. Make sure you balance your final answer. Cu + feso 4 → 5. Get examples of single replacement reactions and learn how to use the metal reactivity series to predict whether a reaction will occur and the products.
Zinc + Hydrogen Chloride Æ 2.
Metal can replace a metal ion in a salt, or a hydrogen ion in an acid: The starting materials are always pure elements like hydrogen gas or a pure zinc metal, plus an aqueous compound. Lead ii chloride + magnesium. React metals with dilute acid to produce hydrogen gas.
These High School Chemistry Worksheets Are Full Of Pictures, Diagrams, And Deeper Questions Covering All Aspects Of Chemical Reactions!
Using the activity series table, complete the following reactions by writing the products that are formed. Web the equation for the reaction is: If the reaction will not occur, write “no reaction.” • if the reaction occurs, write the correct formulas for the reactants and products. Zn + hcl zncl 2 + h 2 type of reaction:
Web single and double replacement reactions practice ws coleman; Chemistry identify each reaction as either single replacement or double replacement and then balance the reactions. If no single replacement reaction occurs, write nr to the right of the arrow. Web fe + cu(oh)2 → write the reactions and predict the products of each of the following single replacement reactions. If no reaction occurs write n.r.