One element and a compound containing that element. _______________________ ca(oh)2 + h3n type of reaction: Cu + ai 2 (so 4) nr 11. Web single replacement reaction worksheet for each reaction use the activity series to complete the reaction. You probably have a composition reaction.
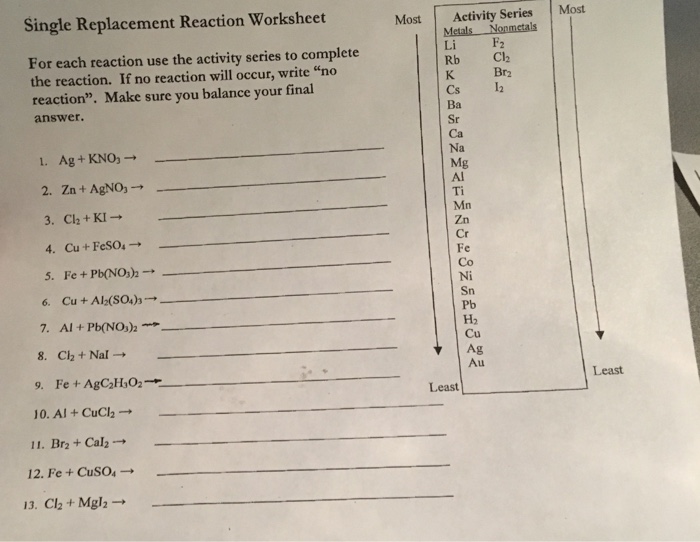
Ag + kno, → 2 zn + agno3 → 3. Zncl2 + h2 type of reaction: A single replacement reaction or a single displacement reaction is defined as a reaction in which one element is superseded by another element in a compound. If there is no reaction, then just put no rxn.
The starting materials are always pure elements like hydrogen gas or a pure zinc metal, plus an aqueous compound. 2al + 3h 2 so 4 3 h 2 + al 2 (so 4) 3 4. If no reaction will occur, write “no reaction”.
Make sure you balance your final answer. Ag + kno, → 2 zn + agno3 → 3. Web single replacement reaction worksheet key | pdf | chemical compounds | sets of chemical elements. If there is no reaction, then just put no rxn. Web chemistry single replacement reaction worksheet answer key 1.
Magnesium + hydrogen sulfate æ. Definition of single replacement (or single displacement) reactions. The starting materials are always pure elements like hydrogen gas or a pure zinc metal, plus an aqueous compound.
A + Bc → B + Ac Or A + Bc → C + Ba (When A And C Are Negative Ions) Zinc + Hydrogen Chloride Æ.
A + b ab [ element1 + element2 compound ] The answer key has detailed answers for every problem that includes each step in finding the. Predict the products and balance the following single replacement reactions. Water and a nonmetal oxide.
Cu + Ai 2 (So 4) Nr 11.
2na + 2hoh h 2 + 2naoh 8. Predicting and determining the products using the reactivity series. Metal can replace a metal ion in a salt, or a hydrogen ion in an acid: Cl 2 + ki → 4.
Cl 2 +2 Ki I 2 + 2Kcl 5.
If no reaction occurs write n.r. One element and a compound containing that element. Zn + 2agno 3 2ag + zn(no 3) 2 3. Included in the chemistry instructor resources subscription.
Ag + Kno 3 Nr 2.
Be sure to balance each equation. Cu + feso 4 nr 7. Get the solutions to all the problems in this worksheet. When fe replaces cu in the following reaction, what will the ionic compound be that forms between fe and so 4 ?
If the reaction will not occur, write “no reaction.” • if the reaction occurs, write the correct formulas for the reactants and products. What is a single replacement reaction? Use the activity series to determine this. You probably have a composition reaction. Magnesium + hydrogen sulfate æ.