Web draw the zwitterion structure for each of the following amino acids. Find compounds which contain this structure. Major species at ph 7.3. An amino acid zwitterion arising from transfer of a proton from the carboxy to the amino group of methionine; Major miscrospecies at ph 7.3.
From german zwitter [ˈtsvɪtɐ] ' hermaphrodite '), also called an inner salt or dipolar ion, [1] is a molecule that contains an equal number of positively and negatively charged functional groups. Find compounds which resemble this structure. Web draw the zwitterion form of a given amino acid. Web methionine (abbreviated as met or m;
Find compounds which resemble this structure. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. At ph 7, the carboxyl group (cooh)(cooh) of methionine is unprotonated (coo−)(coo−) and the amino group (nh2)(nh2) is protonated (nh+3).(nh3+).
Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. At ph 7, the carboxyl group (cooh)(cooh) of methionine is unprotonated (coo−)(coo−) and the amino group (nh2)(nh2) is protonated (nh+3).(nh3+). Methionine zwitterion is an amino acid zwitterion arising from transfer of a proton from the carboxy to the amino group of methionine; Computed by pubchem 2.2 (pubchem release 2021.10.14) dates. At certain ph, amino acids acids can exist in solution in a way such that no net charge exists on the molecule.
Major species at ph 7.3. Find compounds which contain this structure; It is a tautomer of a methionine.
This Entity Has Been Manually Annotated By The Chebi Team.
Find compounds which contain this structure; Modify the amino acid by adding or removing atoms or bonds, and by adding charges where appropriate. It is a tautomer of a methionine. If you make a mistake or wish to reset the image, click on the red arrows in the molecule menu.
Major Species At Ph 7.3.
It contains a carboxyl group (which is in the deprotonated −coo − form under biological ph conditions), an amino group (which is in the protonated −nh+. Supplier information download molfile xml sdf: Determine the charge on an amino acid when it is not at the isoelectric point. This structure can be referred.
Stars This Entity Has Been Manually Annotated By The Chebi Team.
Find compounds which resemble this structure. This entity has been manually annotated by the chebi team. The zwitterion form of an amino acid has both a positive charge and a negative charge, but the overall molecule is electrically neutral. Identify structural components of an amino acid.
Account For Some Of The Typical Properties Of Amino Acids (E.g., High Melting Points, Solubility In Water) In Terms Of Zwitterion Formation.
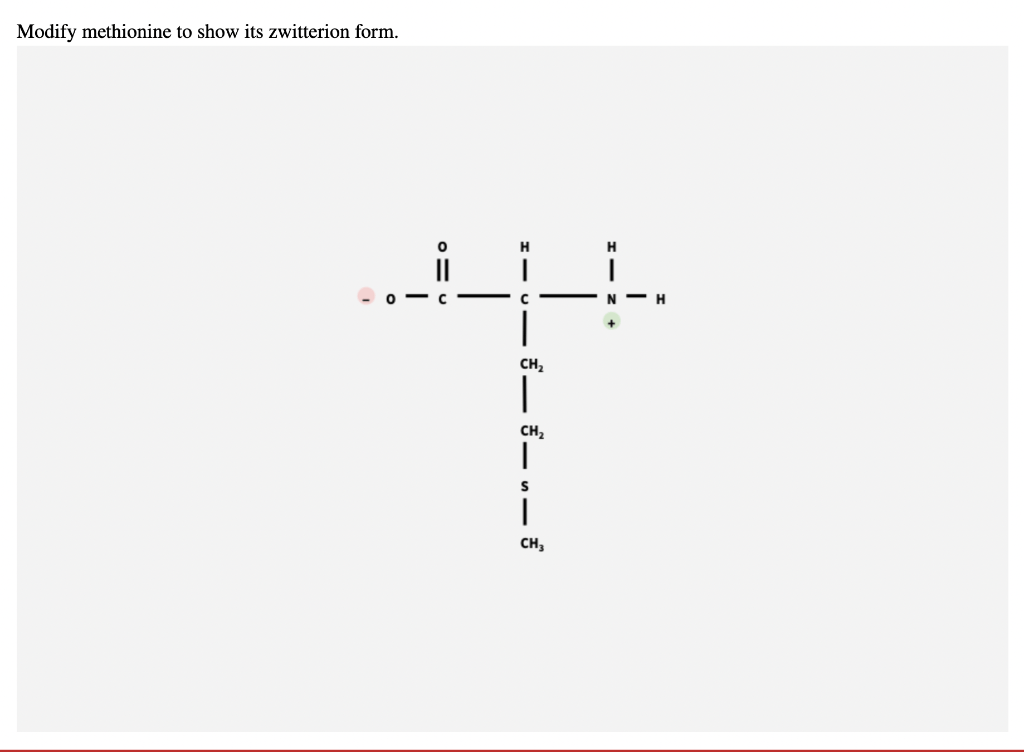
Web draw the zwitterion structure for each of the following amino acids. Modify methionine, below, to show its zwitterion form. Label amino acids as polar and nonpolar and as acidic, basic, or neutral. Modify methionine to show its zwitterion form.
If you make a mistake or wish to reset the image, click on the red arrows in the molecule menu. Major species at ph 7.3. Write appropriate equations to illustrate the amphoteric nature of amino acids. Negative on o and positive on n a zwitterion has equal numbers of positive and negative charges. It contains a carboxyl group (which is in the deprotonated −coo − form under biological ph conditions), an amino group (which is in the protonated −nh+.