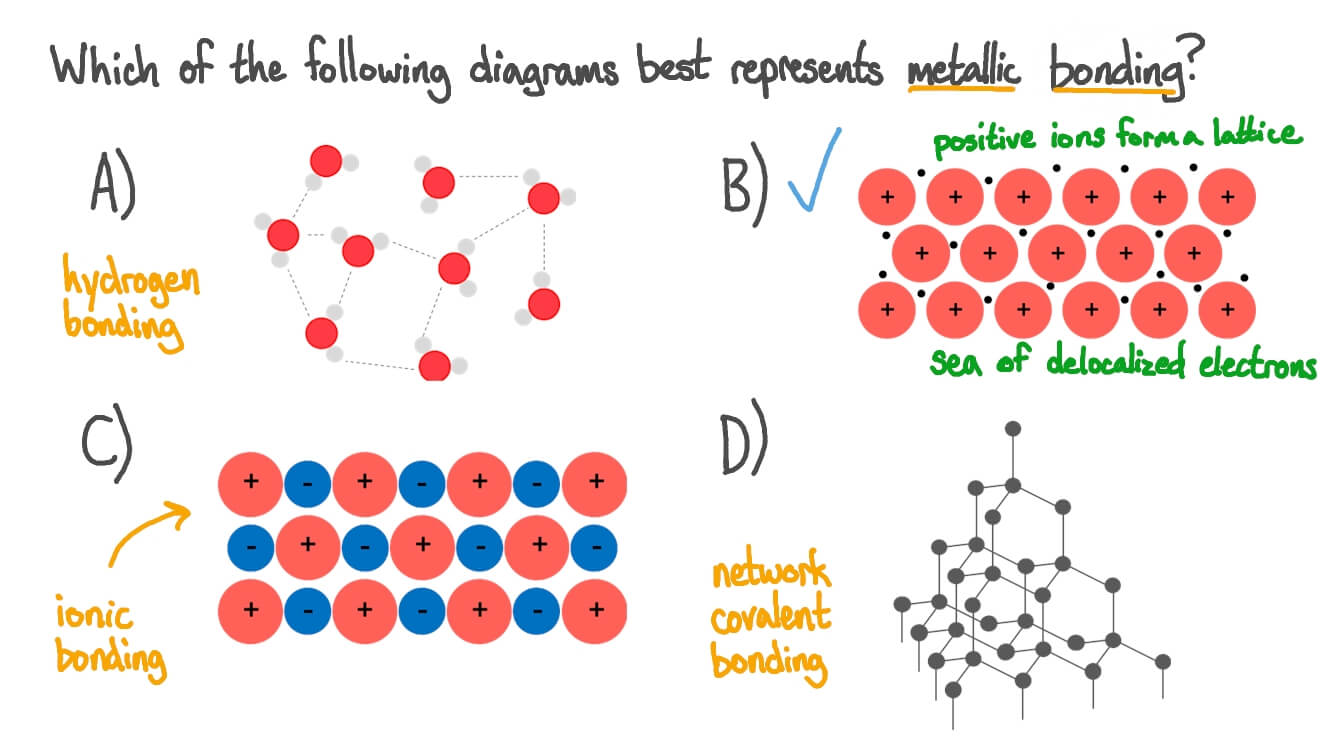
This is because they consist of layers of ions that. Delocalised electrons are free to move throughout. Web metallic bonding is viewed as a sea of free electrons surrounding positive ion cores. Web metallic bonding is a special type of bonding that holds the metals together in metal crystal. ‘metallic bond’ is a term used to describe the collective sharing of a sea of valence electrons between several positively charged metal ions.
The structure and bonding in a substance are. When there are many of these cations, there are also lots of electrons. Metallic bonding is a type of strong chemical bond that occurs in pure metals and alloys. Diagram showing metallic lattice structure with delocalised electrons.
The structure and bonding in a substance are. When there are many of these cations, there are also lots of electrons. When two oxygen atoms bond, they become a molecule and don’t interact much with other molecules.
Metallic bonding is a type of strong chemical bond that occurs in pure metals and alloys. The positive ion cores are attracted to the free electrons. Web metallic bonds are strong and require a great deal of energy to break, and therefore metals have high melting and boiling points. Metallic bonding in transition elements. This bond is neither covalent nor ionic.
Metals tend to form cations. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Solidify your students’ understanding of the structure and properties of metals and alloys.
Even A Metal Like Sodium (Melting Point 97.8°C) Melts At A Considerably Higher Temperature Than The Element (Neon) Which Precedes It In The Periodic Table.
It's like ionic bonding but with a sea of electrons. Metals tend to form cations. There are several theories to explain this type of bonding, among them the electron sea model is most popular. Metallic bonding in transition elements.
A Nineteenth Century Copper Plate.
Metallic bonding is bonding between metal ions in a metal. An ion is an atom (or group of atoms) with a positive or negative charge, formed by either losing or gaining electrons. When there are many of these cations, there are also lots of electrons. Solidify your students’ understanding of the structure and properties of metals and alloys.
Metal Atoms Are Tightly Packed Together In Lattice Structures.
Chemical bonding and molecular structure. When the metal atoms are in lattice structures, the electrons in their outer shells are free to move throughout the structure. The arrangement of the atoms in a metal. You can think of the free electrons as a glue, holding the positive ion cores together.
Web What Is A Metallic Bond?
Metallic lattices do not contain fixed. Metals have tendency to give up electrons and none is their to accept it. Web metallic bonding occurs in metallic elements and alloys. Introductory, conceptual, and gob chemistry.
It can be described as the sharing of free electrons among a lattice of positively charged metal ions. Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. When the metal atoms are in lattice structures, the electrons in their outer shells are free to move throughout the structure. It may be described as the sharing of free electrons among a structure of positively charged ions ( cations ). The positive ion cores are attracted to the free electrons.