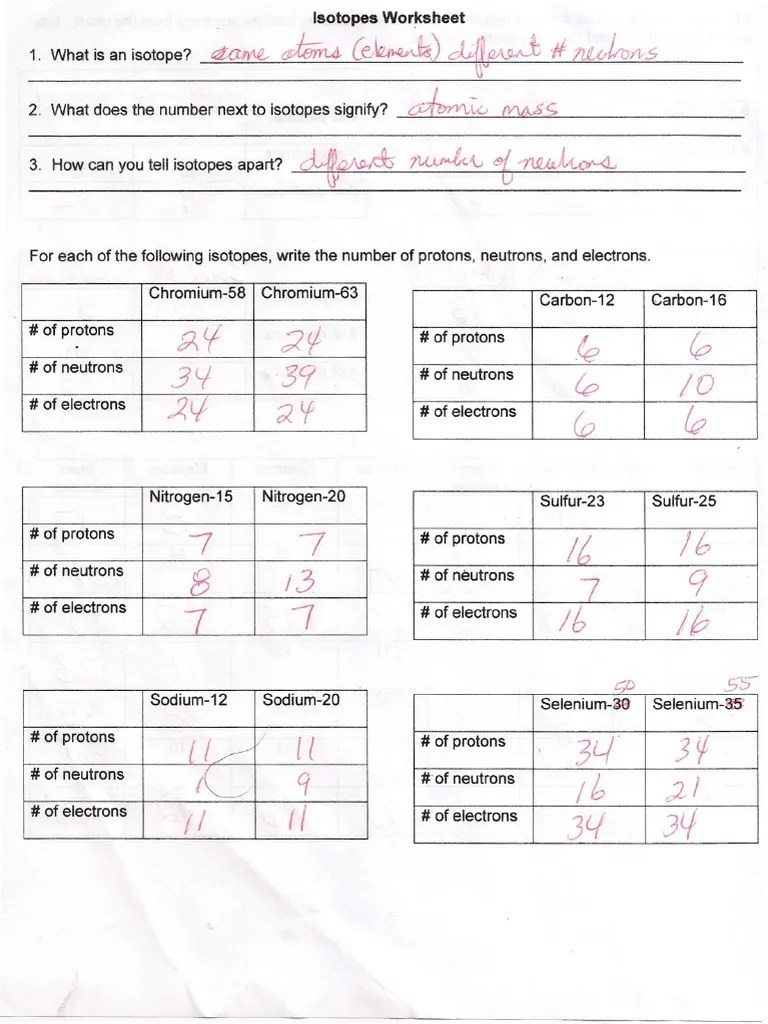
Web atoms of the same element with different numbers of neutrons are called isotopes. How can you tell isotopes apart? Web are all atoms of an element the same? 32 protons, 38 neutrons, 32 electrons. The average atomic mass of a lead atom is 207.2 amu.
A short worksheet to introduce or revise isotopes. How many protons and neutrons are in the first isotope? 32 protons, 38 neutrons, 32 electrons. The number 6 refers to the atomic number.
Web isotopes work sheet. Here are three isotopes of an element: Consider the following illustrations in which protons are shown as pink spheres and neutrons are shown as green spheres.
Which isotope of lead is likely to be the most abundant? The relative atomic mass (ar) of atoms is the average mass of all the different isotopes of an element (taking into account the amount of each isotope) on a scale where 12c atoms have a mass of exactly 12. A short worksheet to introduce or revise isotopes. The average mass of the balls is given by: Imagine you have 90 balls with mass 200 g, and 10 balls with mass 300 g.
Web in a sample of e there are two isotopes. How many protons and neutrons are in the first isotope? The number 6 refers to the atomic number.
These Isotopes Are Neutral (Charge = 0).
Full lesson for gcse chemistry with worksheets on the lesson isotopes and ions. What does the number next to isotopes signify? The numbers 12, 13, and 14 refer to the _____ d. Web isotopes work sheet.
32 Protons, 38 Neutrons, 32 Electrons.
Identify which among the isotope symbols below is incorrect. Web isotope practice worksheet 1. So different isotopes have different mass numbers but the same proton number. Web in a sample of e there are two isotopes.
The Relative Atomic Mass (Ar) Of Atoms Is The Average Mass Of All The Different Isotopes Of An Element (Taking Into Account The Amount Of Each Isotope) On A Scale Where 12C Atoms Have A Mass Of Exactly 12.
Exshare answers from the class, ensuring that all students are able to press 18o/16o in terms of voltage and resistance before allowing them to move on to calculate 18o/16o ratio from the data provided. How many protons and neutrons are in the first isotope? Web ask the class to read through the remaining sections on the provided worksheet, and answer the questions. Here are three isotopes of an element:
How Many Protons And Neutrons Are In The First Isotope?
Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element. The average atomic mass of a lead atom is 207.2 amu. Web worksheets for a lesson on isotopes, including a full set of answers on powerpoint slides. Web are all atoms of an element the same?
If we know the number mass number and the atomic number, we can calculate the number of neutrons in the atom using: A short worksheet to introduce or revise isotopes. Which isotope of lead is likely to be the most abundant? One isotope has a mass number of 10 and the other isotope has a mass number of 11. How many protons and neutrons are in the first isotope?