Consider the following illustrations in which protons are shown as pink spheres and neutrons are shown as green spheres. How many protons and neutrons are in the second isotope? 19 protons, 22 neutrons, 19 electrons. What do all isotopes of an element have in common? The numbers 12, 13, and 14 refer to the mass number.
\textcolor {f21cc2} {\text {number of neutrons}=\text {mass number. The numbers 12, 13, and 14 refer to the ________________________ d. Students shared 1007 documents in this course. Web for each isotope shown, give the number of protons, neutrons, and electrons.
5 protons, 6 neutrons, 5 electrons. What do all isotopes of an element have in common? How can you tell one isotope from another?
For example, the atomic mass of carbon is reported as 12.011 amu (atomic mass units). Consider the following illustrations in which protons are shown as pink spheres and neutrons are shown as green spheres. Protons and neutrons are in the nucleus. The numbers 12, 13, and 14 refer to the mass number d. Isobars are nuclides of different elements (different \(z\)) with the same mass number (\(a\)).
Explain, in terms of subatomic particles, what is meant by the term isotopes. The numbers 12, 13, and 14 refer to the mass number d. Here are three isotopes of an element:
Use The Sim To Learn About Isotopes And How Abundance Relates To The Average Atomic Mass Of An Element.
How many protons and neutrons are in the second isotope? The numbers 12, 13, and 14 refer to the mass number. 6 protons & 6 neutrons e. What does the number next to isotopes signify?
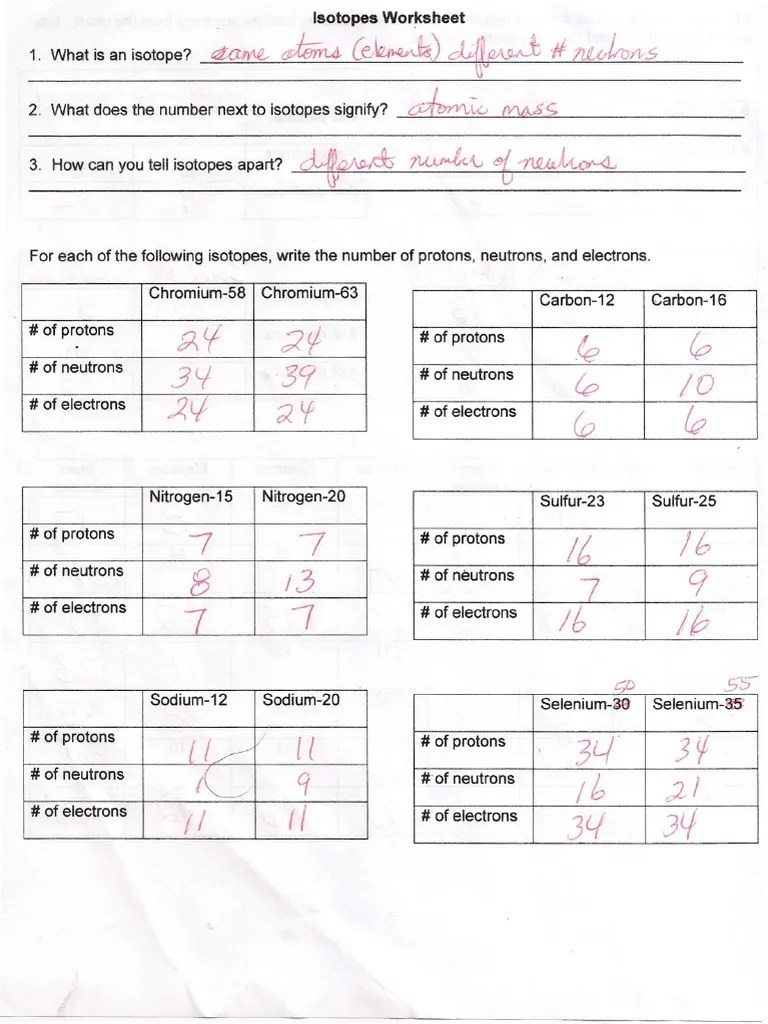
The Numbers 12, 13, And 14 Refer To The ________________________ D.
Web isotope practice worksheet 1. Here are three isotopes of an element: 19 protons, 22 neutrons, 19 electrons. Web isotopes of an element have the same atomic number (z) but have different mass numbers (a), because they have different numbers of neutrons.
The Number 6 Refers To The Atomic Number C.
Web isotopes aqa gcse. 5 protons, 6 neutrons, 5 electrons. For each of the following isotopes, write the number of protons, neutrons, and electrons. \textcolor {f21cc2} {\text {number of neutrons}=\text {mass number.
12C 14 13C C A.
Makes an effective homework or revision resource including a challenge question to stretch higher ability students. If we know the number mass number and the atomic number, we can calculate the number of neutrons in the atom using: Web isotopes worksheets and full answers. Explain, in terms of subatomic particles, what is meant by the term isotopes.
Students shared 1007 documents in this course. Here are three isotopes of an element: One isotope has a mass number of 10 and the other isotope has a mass number of 11. How can you tell isotopes apart? How many protons and neutrons are in the second isotope?