Howstuffworks | aug 25, 2023. Web pyrite, or iron disulfide, is the most common sulfide mineral on the earth’s surface and is widespread through the geological record. These compounds were formed after igneous rocks are pressurized, heated, and subsequently changed over millions of years. Pyrite forms under reducing conditions, typically in environments with low oxygen levels, high sulfur content, and abundant iron. Iron sulfide, sometimes containing small amounts of cobalt, nickel, silver, and gold.
To the novice its colour is deceptively similar to that of a gold nugget. Web how does pyrite form (pyritisation)? Web pyrite typically forms in cubic crystals, often accompanied by striations on the crystal faces. One unexpected consequence of the model is that it further explains how commonly observed groups of framboids can form contemporaneously.
Web the formation of pyrite starts with chlorite iron deposits within the ground. It forms at high and low temperatures and occurs, usually in small quantities, in igneous, metamorphic, and sedimentary rocks worldwide. Often mistaken for gold due to its metallic luster, pyrite has a playful nickname but holds real value.
If there is iron present, iron sulphide crystals begin to grow. Web how does pyrite form (pyritisation)? Web it forms when iron and sulfur combine in specific geological conditions that typically involve the presence of water and oxygen. Pyrite is called fool’s gold; Web the formation of pyrite crystals depends mainly on the iron content of the sediment.
The name comes from the greek word pyr, “fire,” because pyrite emits sparks when struck by metal. Web pyrite forms large bodies in moderate to high temperature hydrothermal deposits and in contact metamorphic ore deposits, is an accessory in many igneous rocks, and is common in sedimentary beds and metamorphosed sediments. Web it forms when iron and sulfur combine in specific geological conditions that typically involve the presence of water and oxygen.
Bisulphite Can Be Partially Oxidised Or Can React With Organic Matter And Reactive Metal Species.
Slower cooling results in larger crystals. Web the formation of pyrite crystals depends mainly on the iron content of the sediment. It is mined as a source of sulfur and iron as well as of the impurities gold and copper. Web the formation of pyrite starts with chlorite iron deposits within the ground.
Web How Does Pyrite Form (Pyritisation)?
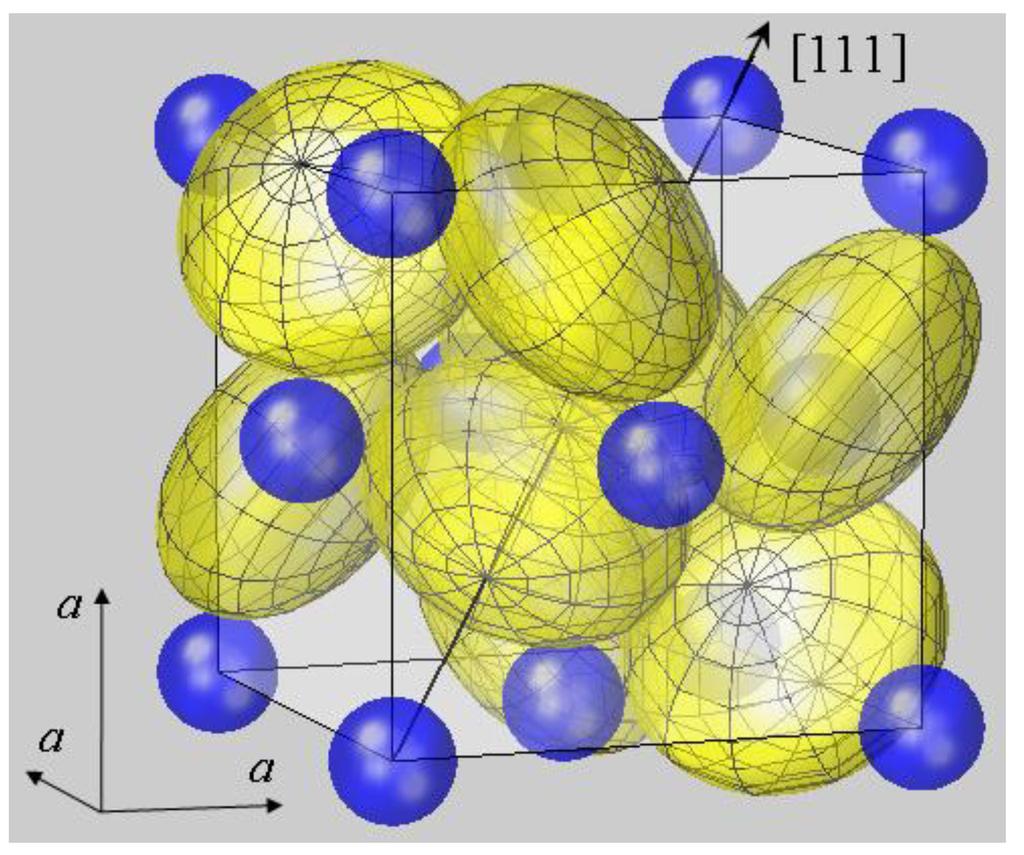
I know that the crystal structure is primitive cubic, but i don’t see how cubic structure on the molecular level translates to cubic structure on much larger scales. Web pyrite, or iron disulfide, is the most common sulfide mineral on the earth’s surface and is widespread through the geological record. It forms at high and low temperatures and occurs, usually in small quantities, in igneous, metamorphic, and sedimentary rocks worldwide. Howstuffworks | aug 25, 2023.
The Specimen’s Size Can Vary.
Pyrite forms under reducing conditions, typically in environments with low oxygen levels, high sulfur content, and abundant iron. Web it forms when iron and sulfur combine in specific geological conditions that typically involve the presence of water and oxygen. It forms in a variety of geological settings through several processes. Web where it is found in igneous rocks, it may have been formed from magma in which the minerals were heated into a melted mass, and then the various minerals separated out at different temperatures, forming crystals as it cooled;
This Then Reacts With An Iron Compound Forming Pyrite.
To the novice its colour is deceptively similar to that of a gold nugget. These chlorite compounds contain the iron found in pyrite, but they also contain silicone and oxygen, which must be removed and. It has a chemical composition of iron sulfide (fes 2) and is the most common sulfide mineral. Iron sulfide, sometimes containing small amounts of cobalt, nickel, silver, and gold.
Web pyrite, or iron disulfide, is the most common sulfide mineral on the earth’s surface and is widespread through the geological record. These crystals can also be octahedral and pyritohedral in shape. The name comes from the greek word pyr, “fire,” because pyrite emits sparks when struck by metal. Over time, iron sulfide minerals crystallize, resulting in pyrite’s formation. These compounds were formed after igneous rocks are pressurized, heated, and subsequently changed over millions of years.