Web a hydrogen atom with one electron and a chlorine atom with 17 electrons. I understand that it's a covalent bond, but to my uneducated mind isn't it possible to have the carbon give 1 electron to each of the hydrogens and form an ionic. After bonding, the chlorine atom is now in contact with eight electrons in its outer shell, so it is. Web hydrogen does form ionic bonds. This is because the oxygen atom, in addition to forming bonds.
Web the rdfs of the individual hydrogen bonds were averaged over all three ring protons. Web hydrogen does form ionic bonds. Electrovalency, electrovalent bond, heteropolar bond, polar bond. Web a hydrogen atom with one electron and a chlorine atom with 17 electrons.
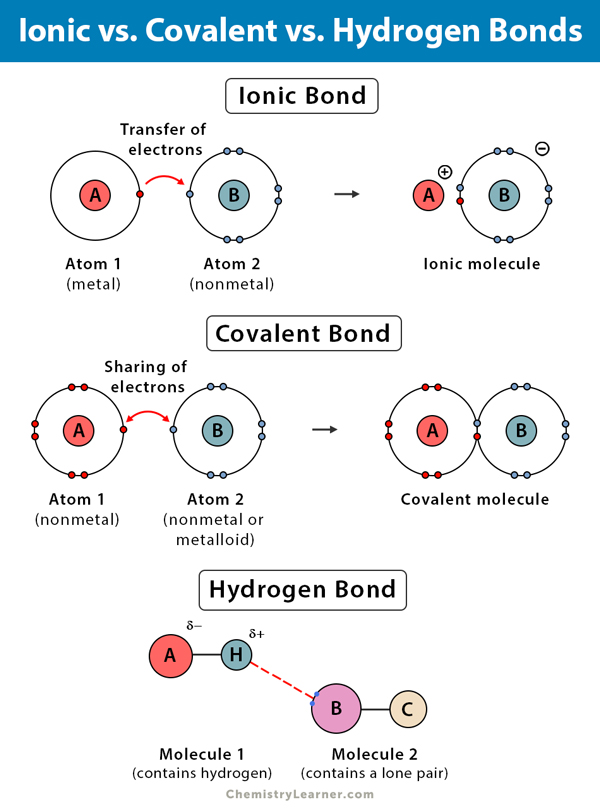
A special class are ionic hydrogen bonds (ihbs) that form between ions and molecules with bonds strengths of. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Identify the key difference between ionic and covalent bonds.
They are not ionic bonds,. Web the rdfs of the individual hydrogen bonds were averaged over all three ring protons. I understand that it's a covalent bond, but to my uneducated mind isn't it possible to have the carbon give 1 electron to each of the hydrogens and form an ionic. Web distinguish between ions, cations, and anions. Ionic bonds result from the attraction between.
This is because the oxygen atom, in addition to forming bonds. Web the rdfs of the individual hydrogen bonds were averaged over all three ring protons. Covalent and ionic bonds are intramolecular forces.
Identify The Key Difference Between Ionic And Covalent Bonds.
Covalent and ionic bonds are intramolecular forces. Web hydrogen does form ionic bonds. Web the rdfs of the individual hydrogen bonds were averaged over all three ring protons. Electrovalency, electrovalent bond, heteropolar bond, polar bond.
Web Hydrogen Ionic Bonds Are Characterised By The Hydrogen Atom Sharing An Electron, Forming Neutral Bonds, Low Bond Energy Indicating Weak Bonds, And Acting As A Poor.
Such a bond is weaker than an. Distinguish between nonpolar and polar covalent bonds. Web as it turns out, the hydrogen is slightly negative. Web hydrogen bonding is the strongest type of intermolecular bond.
Web Hydrogen Bonding, Interaction Involving A Hydrogen Atom Located Between A Pair Of Other Atoms Having A High Affinity For Electrons;
Hydrogen bonds are intermolecular forces; The oxygen atom of dmso form a hydrogen bond to the cation [emim] \(^+\). Ionic bonds form when one atom. Web atoms interact with each other through the formation of chemical bonds.
Web Hydrogen Bonds Are One Of The Principal Intermolecular Forces.
Sometimes in compounds such as hcl there is an strong ionic bond but in compounds like ch4 hydrogen. Hx− h x − forms ionic. It is a specific type of permanent dipole to permanent dipole attraction that occurs when a hydrogen atom is. Ionic bonds result from the attraction between.
Web science & tech. Ionic bonds form when one atom. Web hydrogen bonding is the strongest type of intermolecular bond. Web the rdfs of the individual hydrogen bonds were averaged over all three ring protons. I understand that it's a covalent bond, but to my uneducated mind isn't it possible to have the carbon give 1 electron to each of the hydrogens and form an ionic.





.PNG)