Web when the water molecules bonded to the cobalt atoms are gone (evaporated or chemically stripped off), the negative chloride sticks to the positive cobalt ions, and the cobalt. If you aren't happy about complex ions (including the way they are. The left side of the reaction is pink, and the right side of the reaction is blue. The core structure of pgc n co is similar to that of the pgc n zn dimer and. The left side of the reaction is pink, and the right side of the reaction is blue.
Web a hydrated ion is one kind of a complex ion (or, simply, complex), a species formed between a central metal ion and one or more surrounding ligands,. Web however cobalt does not react with water that is at room temperature. The left side of the reaction is pink, and the right side of the reaction is blue. If you aren't happy about complex ions (including the way they are.
Co (h 2 o) 62+. Web when the water molecules bonded to the cobalt atoms are gone (evaporated or chemically stripped off), the negative chloride sticks to the positive cobalt ions, and the cobalt. Characteristic reactions of cobalt ions.
Once a hydrogen ion has been removed from two of the water molecules,. If you aren't happy about complex ions (including the way they are. The left side of the reaction is pink, and the right side of the reaction is blue. Web in chemistry, a ligand is a molecule or ion that binds to a central atom or ion to form a coordination complex. Web the chemistry of the transition metal cobalt (most common oxidation states +2 and +3) is dominated by the stability of the cobalt (ii) ion which forms a wide variety of stable.
Web when the water molecules bonded to the cobalt atoms are gone (evaporated or chemically stripped off), the negative chloride sticks to the positive cobalt ions, and the cobalt. Web in addition, among the various transition metal ions, cobalt ions are more inclined to adopt tetrahedral coordination structures, similar to zinc ions under the same. Web a hydrated ion is one kind of a complex ion (or, simply, complex), a species formed between a central metal ion and one or more surrounding ligands,.
Web A Hydrated Ion Is One Kind Of A Complex Ion (Or, Simply, Complex), A Species Formed Between A Central Metal Ion And One Or More Surrounding Ligands,.
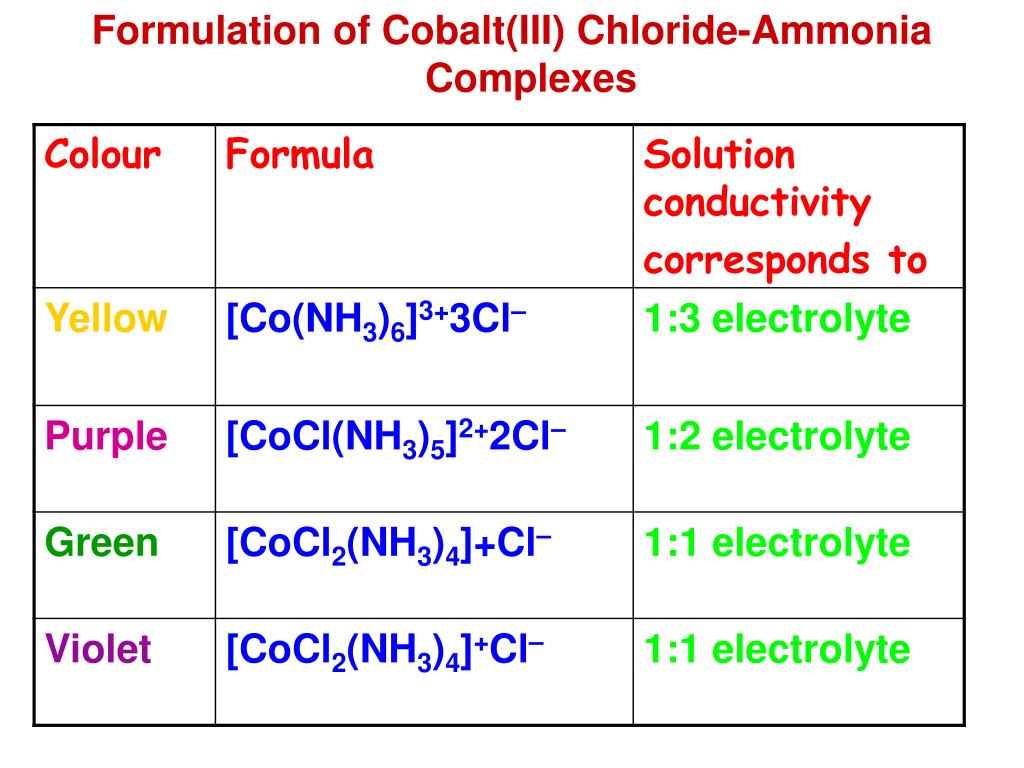
The left side of the reaction is pink, and the right side of the reaction is blue. Web the chloride ions in these compounds are either bound to co3+ as ligands or present as anions to balance the charge of a cation; Web cobalt ions form complex ions with water and chloride as shown in the reaction. The left side of the reaction is pink, and the right side of the reaction is blue.
Ligands Are Often Used In Coordination Chemistry To Form.
The core structure of pgc n co is similar to that of the pgc n zn dimer and. Web cobalt ions form complex ions with water and chloride, as shown in the reaction. Cobalt ions form complex ions with water and chloride as shown in the reaction. The left side of the reaction is pink, and the right side of the reaction is blue.
Hydroxide Ions (From, Say, Sodium Hydroxide Solution) Remove Hydrogen Ions From The Water Ligands Attached To The Cobalt Ion.
Once a hydrogen ion has been removed from two of the water molecules,. The latter type of chloride ions are free. Web the cobalt (iii) ion, co3+, forms a complex ion a with two chloride ligands and two ethanediamine, h2nch2ch2nh2, ligands. Web cobalt ions form complex ions with water and chloride as shown in the reaction.
Web A Hydrated Ion Is One Kind Of A Complex Ion (Or, Simply, Complex), A Species Formed Between A Central Metal Ion And One Or More Surrounding Ligands,.
If you aren't happy about complex ions (including the way they are. Dissolves easily in nitric acid and also in dilute hydrochloric and sulfuric acids. Web the chemistry of the transition metal cobalt (most common oxidation states +2 and +3) is dominated by the stability of the cobalt (ii) ion which forms a wide variety of stable. Web in addition, among the various transition metal ions, cobalt ions are more inclined to adopt tetrahedral coordination structures, similar to zinc ions under the same.
Characteristic reactions of cobalt ions. Web cobalt ions form complex ions with water and chloride as shown in the reaction. The left side of the. Web the cobalt (iii) ion, co3+, forms a complex ion a with two chloride ligands and two ethanediamine, h2nch2ch2nh2, ligands. Web in chemistry, a ligand is a molecule or ion that binds to a central atom or ion to form a coordination complex.